Introduction
The periodic table is a critical tool in chemistry, providing a systematic arrangement of chemical elements based on their atomic number, electron configurations, and recurring chemical properties. Its significance extends beyond the laboratory, influencing various fields such as medicine, engineering, and environmental science. Recognising its relevance not only aids in academic study but also enhances public understanding of the natural world.
History and Development
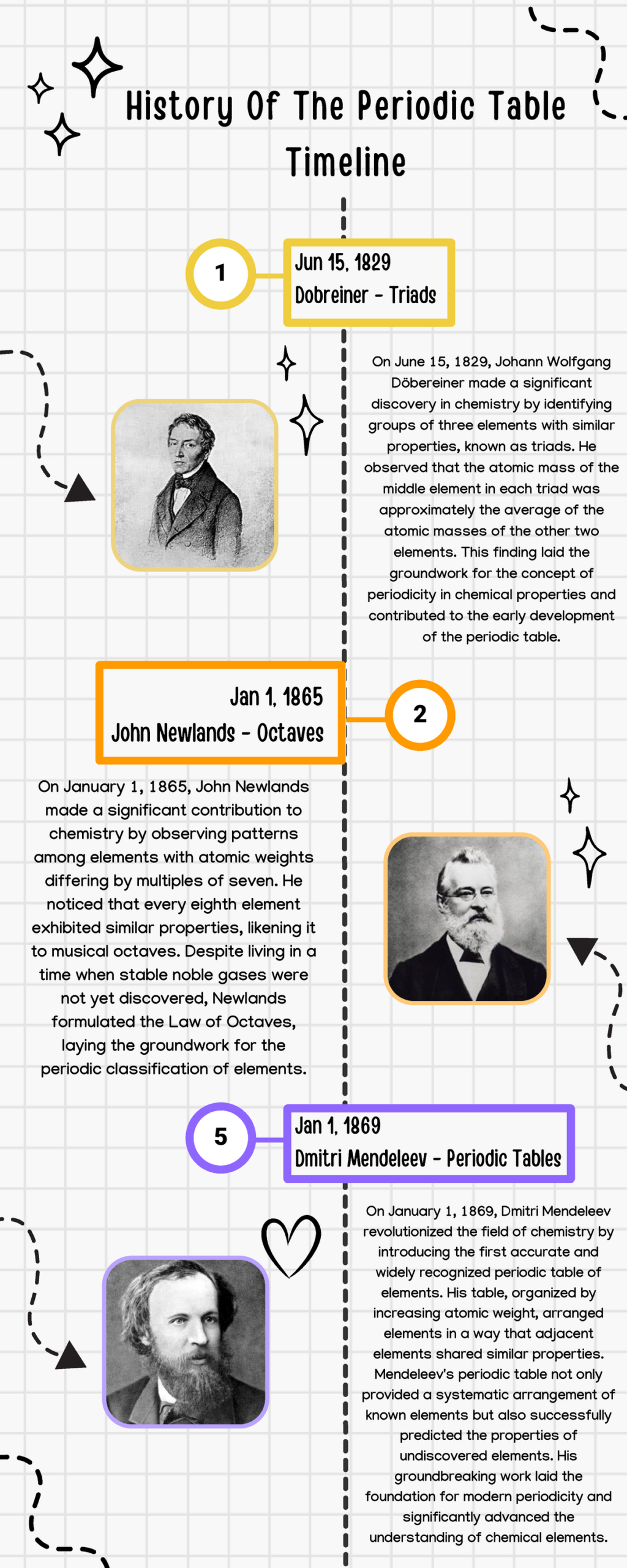
The concept of the periodic table was first introduced in 1869 by Russian chemist Dmitri Mendeleev, who organised the elements by their atomic mass and predicted the existence of undiscovered elements. Over time, the table has evolved, with the modern layout developed through the discovery of atomic structure and the periodic law, which states that elements with similar properties occur at regular intervals when arranged by atomic number. Today, 118 confirmed elements populate the periodic table, ranging from hydrogen, the lightest and simplest element, to oganesson, a synthetic element that is among the heaviest known.
Importance in Science and Education
The periodic table is not merely an assemblage of symbols and numbers but a comprehensive guide that allows scientists to predict the types of chemical reactions that substances can undergo. For students, it serves as a foundational tool in various scientific disciplines, helping learners grasp elemental properties, atomic structure, and the relationships between different elements. Recent educational reforms emphasise the importance of integrating interactive periodic tables into learning environments, making chemistry more accessible and engaging for students. Moreover, its role in STEM education is pivotal, illustrating the connections between different scientific fields.
Recent Developments and Future Prospects
In recent years, research into elements beyond those currently known has become a focal point within materials science and nuclear physics. Scientists are exploring potential new elements and their properties, which could revolutionise technology and materials. Furthermore, the periodic table’s applications are expanding in fields such as nanotechnology, green chemistry, and bioengineering, promising enhanced efficiency and sustainability in future scientific innovations.
Conclusion
<pIn conclusion, the periodic table remains an essential reference in chemistry and science education, influencing research and practical applications in myriad fields. As new discoveries emerge, the periodic table will continue to evolve, reflecting the dynamic nature of scientific exploration and its profound impact on technology and the environment. For readers, understanding the periodic table not only enhances knowledge of chemistry but also enriches appreciation for the interconnectedness of scientific disciplines.